|
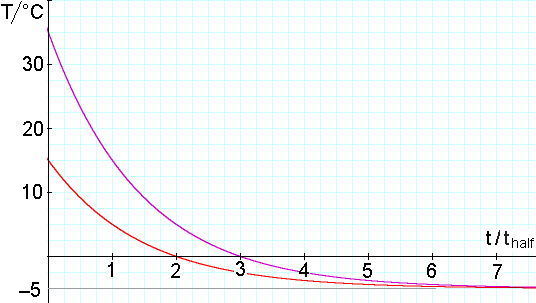 The next example shows why. Again, suppose that our freezer is at − 5.0°C. Further, let’s suppose that one sample (A) freezes at − 4.5°C (ΔT = 0.5°C) and another (sample B) freezes at − 4.9°C (ΔT = 0.1°C). The second sample’s freezing temperature is four times closer to the freezer temperature so, if the two samples started from the same initial temperature, the second will remain unfrozen for longer, by a time of 2*thalf. The next example shows why. Again, suppose that our freezer is at − 5.0°C. Further, let’s suppose that one sample (A) freezes at − 4.5°C (ΔT = 0.5°C) and another (sample B) freezes at − 4.9°C (ΔT = 0.1°C). The second sample’s freezing temperature is four times closer to the freezer temperature so, if the two samples started from the same initial temperature, the second will remain unfrozen for longer, by a time of 2*thalf.
Now, remember that it takes only one thalf for the samples to cool from 35 to 15°C. So sample B could start at 35°C and sample A from 15°C but sample B would freeze first. This is the situation depicted in the graph.
Why would the freezing temperatures be different? It could be chance: perhaps one sample has more effective ice nucleators than the other – and remember that it only takes one nucleator to initiate freezing. Further, because some of the nucleators are biochemicals, it is possible that even moderate of heating one of the solutions might change the effectiveness of the nucleators.
| 


