Why light? Why vision?
Less than one octave of one type of radiation (electromagnetic) is responsible for so much of what we know about the world – not to mention the universe. Visible light drives photosynthesis, which produced the Earth's oxygen-rich atmosphere and still influences climate in several ways. Light has had a huge effect on evolution: because even primitive sensitivity to light is valuable, eyes have evolved independently many times, sometimes with divergent principles (e.g. vertebrates vs insects), sometimes converging to fairly similar structures (vertebrates and molluscs). Mark's interest was mainly biological, but his question encouraged me to think about the basic physics of vision from a new perspective.
Other possibilities. First, there are other ways of obtaining information about the non-adjacent world. For some sources, there's chemical assay, i.e. smell, if the wind is in the right direction. Sub-atomic particle beams aren't good for us and don't tell us about radioactive sources until it's too late. Mechanical waves are possible if the source moves and is sufficiently powerful: earthquakes, for example, and perhaps the footsteps of large animals.
Sound. Sound is an important alternative, of course: some things of evolutionary interest (including other humans) emit sound, which can carry detailed information. For silent objects, some animals (e.g. bats and blind humans) use echo-location – while echo-location with light is something that humans learned to do when we mastered fire. So let's compare hearing and vision in detail later.
Other impossibilities: But what other waves are there? Since 2016, gravity waves have delivered brief but very interesting information about the orbits and fusion of black holes and neutron stars. We're hoping that they'll also tell us about the early universe. (By early, I mean in its first 400,000 years, which we can't see because in the early universe there were no neutral atoms, just ions, electrons and photons. Photons from that age are now doppler shifted to become cosmic microwave background). The strong and weak nuclear forces have ranges measured only in femtometres. Neutrinos are great for information about the very early universe, but there are good reasons why detectors are extremely large and extremely insensitive, and therefore not possible in biology.
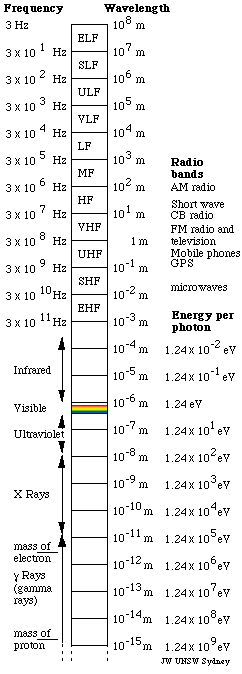
That leaves one remaining force of range greater than nuclear dimensions: electromagnetic waves. Light is a wave of electric and magnetic fields. (See The nature of light.) Its range is limited only by the sensitivity of detectors. But the electromagnetic spectrum is vast: it covers several dozen octaves. Details and the names of different bands are given in the figure at right. So, why this little window of one octave, with wavelengths in the range about 0.4 to 0.7 μm (0.0004 to 0.0007 mm)?
The first answer is: Because it's there! For hours each day, we have a very powerful source of electromagnetic radiation (EMR): radiation from the sun is most intense in the visible, although there is plenty of intensity in the infrared (the sun feels warm) and some in the ultraviolet (it tans). The (current) atmosphere is fairly transparent to visible and the near IR and UV, with a few exceptions. (The foregoing argument is a bit simplistic: in the infrared there is less power at longer wavelengths because there is less energy per photon. If vision were just counting photons, the near IR would be as useful as the visible.)
But there's more to it. I like to make this analogy between water and light: macroscopically, water seems to be continous: we can divide it in half and still have water. Again and again. But, if you start with a litre, after 80 divisions you are down to molecules: on the very small scale, water is lumpy. Likewise light: it seems continuous in the macro-world, but on the small scale, light is lumpy too. It comes in photons. And the molecule-photon interaction is what makes vision possible.
Electromagnetic radiation interacts with electrons: it's only a little misleading to say that it can cause electrons to vibrate. (In principle, it can vibrate protons as well, but these are roughly 2000 times more massive, and are often bound in even more massive nuclei.) Either singly or in groups, photons can transfer energy to the electrons in molecules or in circuits, and molecules and circuits can emit photons or groups of them.
A key point for this story is a conclusion published by Einstein in 1905, the one that won him the Nobel prize. The energy, E per photon is proportional to the frequency, f, or inversely proportional to the wavelength, λ : E = hf = hc/λ, where h is Planck's constant. A photon of violet light (short wavelength) has about twice the energy of a red photon (long). Because one photon can interact with one electron, a useful unit of energy in this case is the electron volt (eV): the work required to increase the voltage (electrical potential) of an electron by one volt. The chart at right shows that visible photons have energies of a couple of eV.
Now convert this to the units of chemistry: one eV for one electron is roughly 100 kilojoules for one mole of electrons (1 eV = 96 kJ/mol). When chemists measure the energies and enthalpies of reactions, they typically get a few hundred kJ/mol: less than that if the chemicals are unstable, more if they are stable. So, to put it informally, a photon of visible light has enough energy to change the shape of a biochemical molecule like rhodopsin, our photopigment, but not enough to tear it apart.
UV vision? Each UV photon, on the other hand, has more energy: enough to damage some biomolecules. And the far UV, or X and gamma rays, have enough energy to break the carbon-carbon bond. So some insects and birds see in the near UV (and so see the patterns in flowers that look homogeneously coloured to us), but no-one sees (unaided) in the far UV.
IR vision? On the other end, some fish see in the near IR: they have photoreceptor molecules sensitive to photons with energies around 1 eV. But there is a disadvantage to IR vision. At ordinary temperatures, molecules are ceaselessly interacting due to their thermal motion. A typical value of the thermal energy of a molecule is about 0.025 eV but, because the motion is random, some molecules have much more energy than this. So photoreceptors in the IR are also more sensitive to thermal noise. So our visible spectrum can be recounted as a Goldilocks story: at much shorter wavelengths, molecular damage is possible; at much longer wavelengths, thermal noise is a problem. Our range is in part the result of evolutionary accident but it could not have been much wider.
One can reverse this argument, too, and say that, at this temperature, there is lots of molecular information to see in the visible range. The far IR only shakes the molecules around, the far UV tears them apart, but the visible probes those molecular resonances that interact specifically with molecules without doing irreversible damage. Similarly, it follows that photosynthesis is a complicated process, because one visible photon does not carry enough energy for a typical biochemical reaction.
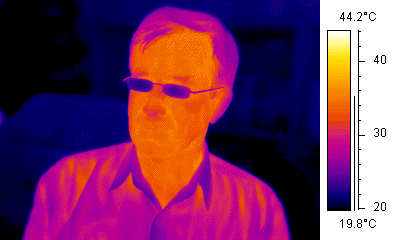 A sense rather different from vision is that of the beetles (e.g. fire beetles) and snakes (including carpet snakes) that can 'see' in the far IR (a broad band around 10 μm or 0.01 eV). One of these photons doesn't have nearly enough energy to excite a photoreceptor molecule. Instead, the snakes' infra-red sensor senses heat: many photons in IR thermal radiation from warm objects heat up cells in regions of the pits on the snake's head, and the cells respond to the heat produced by very many photons adding extra kinetic energy to the tissue molecules. This sense allows some snakes to detect warm objects (potential prey or predators) and cool refuges (useful for animals that control their temperature by moving). We have a crude version of this sense: wave your hand over the stove and you can tell which hotplate is on. The snake uses this sensitivity in a more sophisticated way, because the geometry of the receptor is a bit like a pinhole camera. I don't think that anyone's measured their thermal acuity, but from anatomical considerations we can be confident that their thermal image is not nearly as good as the false-colour IR image at right. (Note that the glasses are cool, in the thermal sense anyway, and opaque to IR at these wavelengths. More about thermal radiation and how clothes work.)
A sense rather different from vision is that of the beetles (e.g. fire beetles) and snakes (including carpet snakes) that can 'see' in the far IR (a broad band around 10 μm or 0.01 eV). One of these photons doesn't have nearly enough energy to excite a photoreceptor molecule. Instead, the snakes' infra-red sensor senses heat: many photons in IR thermal radiation from warm objects heat up cells in regions of the pits on the snake's head, and the cells respond to the heat produced by very many photons adding extra kinetic energy to the tissue molecules. This sense allows some snakes to detect warm objects (potential prey or predators) and cool refuges (useful for animals that control their temperature by moving). We have a crude version of this sense: wave your hand over the stove and you can tell which hotplate is on. The snake uses this sensitivity in a more sophisticated way, because the geometry of the receptor is a bit like a pinhole camera. I don't think that anyone's measured their thermal acuity, but from anatomical considerations we can be confident that their thermal image is not nearly as good as the false-colour IR image at right. (Note that the glasses are cool, in the thermal sense anyway, and opaque to IR at these wavelengths. More about thermal radiation and how clothes work.)
Bio-radar? Going longer in wavelength, we can sense microwave radiation indirectly if it is intense enough and has the right wavelengths to warm up tissue (e.g. microwave ovens), but there are no suitable natural sources: there is even less microwave and radio power in daylight than there is IR. (Radio/microwave telescopes are usually much larger than optical telescopes for two reasons: one is that they need a large ratio of diameter to wavelength to resolve small objects, the other is that the power per unit area radiated by stars is much lower in the radio spectrum than in the visible.) Echolocation in the microwave band is called radar, and there are serious impediments to biological radar. Radio and radar signals are different from most light sources in that the photons emitted in radar or radio are all in phase: their individual electric fields add up to give a strong field that can accelerate crowds of electrons in receiving antennae. Some animals (e.g. platypus) can detect electric fields, and some can create them (e.g. electric eels) but only at extremely low frequencies. So it's hard to imagine bio-radar.
The temperature of the sun, people etc. Oversimplifying somewhat, the wavelength at which an object emits with greatest intensity depends on its temperature in a very simple way: they are inversely proportional (Wien's law: λmaxT = 2.9 mm.K). The sun's (surface) temperature is roughly 5,800 K, which is why, as we noted above, it radiates most strongly in the green part of the spectrum (roughly 0.5 μm). We (surface temperature about 300 K) are nearly 20 times colder, so we radiate most strongly at about 30 μm, which can be detected by the aforementioned snakes.
In the inquisitive spirit of this essay, you'll probably now ask the interesting question: why are we colder than the sun? A simple calculation shows us that the only reason why the sun is hotter than us is because it is bigger. You and I produce thousands of times more energy per kilogram than the sun*. However, heat energy is produced per unit volume (by nuclear fusion and metabolism respectively) but is radiated (and in our case convected) away per unit area. Our ratio of volume to area is a few centimetres, while that of the sun is 230,000 km. So, in spite of our very much higher specific rate of energy production, we are much colder than the sun. (*Depending strongly on what we are doing, we lose say a few hundred watts out of less than 100 kg: so a few watts per kg. The sun's radiation is 1400 watts per square metre at the distance of the earth (r = 8 light minutes) which, multipled by 4πr2, gives 3x1026 W. Divided by the mass of the sun, 2x1030 kg, this gives less than a milliwatt per kg.)
Extraterrestrial vision. If we (Earth based life) were significantly colder, then the thermal noise of our molecules would be proportionately less, so we might have evolved vision in the IR. A life-form on a hotter planet under a hotter star would deal with more thermal noise. It might also perhaps have an alternative biochemistry (silicon instead of carbon?) and so more stable molecules. It might see in the UV. But not very far in the UV, because I don't think that there are any molecules stable at temperatures above a few thousand K. So some of the exoplanets recently discovered are too hot for chemistry, let alone life.
So Mark, while I share your wonder at the power of vision, there is a first, short answer to your question: It's largely because we live near a star whose surface temperature is about 6,000 K on a planet that is about 20 times colder.
We can again ask why. Part of the reason for that is our orbit: overall, the more distant planets are colder and the closer ones hotter. However, in its youth, the earth was very much hotter. First, it was heated by its own gravitational collapse: losing some of the kinetic energy and gravitational potential energy of the components that formed it. Then heated again by later collisions, especially the large one that split off the moon. The earth's radioactivity – a considerable contribution to the surface temperature – was much stronger then. Some claim that life began (under the sea) only a few hundred million years after the earth formed, so it probably must have cooled below the boiling point by then. The atmosphere makes a big difference: a big fraction of the incoming energy is in the visible (~ 0.5 ?m) but the earth radiates it out into the rest of the sky in the far IR, where different atmospheric compositions make a difference. (With the worrying exception of the recent global warming, the solar power absorbed by the earth almost exactly equals the IR power radiated from earth. However, because these occur at different temperatures, there is a huge increase in entropy: details in this chapter.)
Vision vs hearing
Because they both tell us so much about the world, it's interesting to compare the performance of the eye and the ear. Both eye and inner ear are remarkable for their performance, sophistication and small size.
In sensitivity, both eye and ear are within an order or magnitude or two of the theoretical maximum: the eye needs about 70 photons focussed on a single receptive field in one integration time to cause us to see a flash. An ear that was much more sensitive than ours would hear the thermal motion of the air.
In intensity, we have a large range in both. The intensity that quickly causes damage is very roughly a thousand billion times the weakest signal we can detect. So both the ear and the eye have a dynamic range of about 120 dB. (See What is a decibel?) (In addition, both have protective reflexes: blinking protects the eye and the acoustic reflex disconnects the ossicles from the tympanum to protect the ear.)
In frequency range, the ear wins easily: young ears have a range exceeding ten octaves compared with the eye's one. In frequency discrimination, the ear wins too: we can distinguish frequencies that differ by a fraction of a percent. Further, the ear is not fooled by mixtures: red plus green looks yellow, but the note C plus the note E sounds like the chord C plus E: it certainly doesn't sound like D, which lies between them on the spectrum.
In angle, the ears have an advantage: we can hear sounds from all directions, whereas our eyes see only about half the possible directions.
It is in spatial resolution, of course, that the eye has an enormous advantage. We do have some directional sense of hearing, due to arrival times, the sound shadow of the head and the directional variation in frequency response of the outer ear. But this is trivial compared to the performance of the eye. Let me put it thus: if someone seated at an organ sounded all pipes in the display rank simultaneously, we certainly couldn't identify the position of all the pipes. The eye, however, not only sees every pipe, but can tell us the shape and the decoration. This is why the eye needs about one hundred times as many nerve channels as the ear – though the ear does much more signal processing before transmission to the brain.
In response time, events closer than a few tens of milliseconds are hard to distinguish with either sense. The two ears, however, can detect arrival times differing by much less: this is part of our directional hearing.
They use somewhat different information codings. The eye codes both position and colour with different channels: what neurophysiologists call place coding. Intensity is coded by the frequency of pulses, both with a number of interesting complications. The ear uses a mix of place and rate codings for both intensity and pitch.
Unless you live in a cave or at the bottom of the ocean, or are nocturnal, it's easy to be persuaded that an eye confers a very big advantage to potential predators and prey. Unless you live in a noisy environment, it's also easy to understand why hearing confers advantages of similar importance. Which is why we have both. Which is more important? That's hard to say. However, my mother, who is both blind and deaf once said that, if asked to choose, she would rather have her hearing back. 'Vision is for things, hearing is for people', she said.
This page supports the multimedia tutorial The eye and colour vision.
|

