Colours in a soap film
The reflected colours in both the film clip above and the photo below result from white light reflected in a thin film made from water, glycerol (which increases the viscosity) and detergent, which reduces the surface tension (or interfacial free energy) by forming a layer, one molecule thick, on the air-water interface. This reduction in surface tnesion makes the thin film more stable. The detergent layers at the surface also affect the process of draining, as we explain below.
Once the film is formed, the water-glycerol solution between the two detergent layers at the surface
drains downward under gravity, making the film thinnest at the top, as shown in the sketch below right. This leads to the range of colours, as we shall now discuss.
First, consider a region in the top third of the film shown, where it appears black. Here there is no reflected light because of destructive interference. In this region, the thickness is very much less than one wavelength of visible light, as indicated at the top of the sketch. (In fact, the film here is only several molecules thick.) The reflection from the first interface (air towards water) has a phase change of π, while that at the second interface (water towards air) has a phase change of zero. So we have destructive interference and a black film.

Above: a photograph of a draining soap film and the colours produced by constructive and destructive interference, as we explain with reference to the sketch at right. |
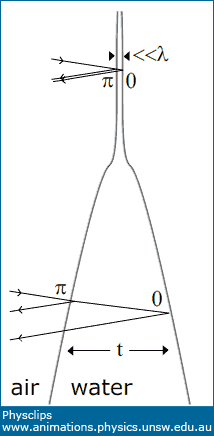 |
Now look
at the yellow band across the middle of the film in the photo. At this point, the thickness t is about half the wavelength of blue light in water (i.e. t ~ λblue/2n). For blue light, the phase difference in reflection has a component of
one complete cycle or 2π due to path length difference, and
π due to the reflections. So there is destructive interference for blue light. Green and red light do not have strong destructive interference, so we see red+green = yellow. (To understand why we consider only red, green and blue, see The eye and colour vision.)
A little below the yellow band (where blue has destructive interference), the film is a little thicker, so there is destructive interference for green light. So we see red+blue = magenta. And somewhat below that, there is a thickness where red light has destructive interference, so we see green+blue = cyan. Then further bands of colours as we reach thicker parts of the film.
Let's repeat those two figures while we discuss a few more details.

Above: a photograph of a draining soap film and the colours produced by constructive and destructive interference, as we explain with reference to the sketch at right. |
 |
Now look at the broad white band, below the black (t << λ) but above the yellow( t ~ λblue/2n). Here, none of the visible wavelengths have destructive interface, so we see red+green+blue = white. It raises the interesting question, however: why is the junction between black and white so sharp? Obviously, the thickness of the film must go rapidly from << λ to a thickness great enough to avoid destructive interference for any wavelengths. What's going on?
I've done no research on this, but do have a background in surface forces, so I'll propose (fairly confidently) this explanation. In the black film region, there are attractive van der Waals forces between the two detergent layers at the surface. These are opposed by repulsive hydration forces between the hydrophilic sides of the detergent layers, which stop them getting any closer (and thus probably destroying the film). These hydration forces have very short range – a characteristic length of about 0.2 nm. The attractive van der Waals forces have much longer range and their attraction squeezes water out from near the black-white boundary. This is still a fairly thin layer, however, so the last layers of water are slow to leave, so there is a bulge just below the black-white boundary, as shown in the sketch.

